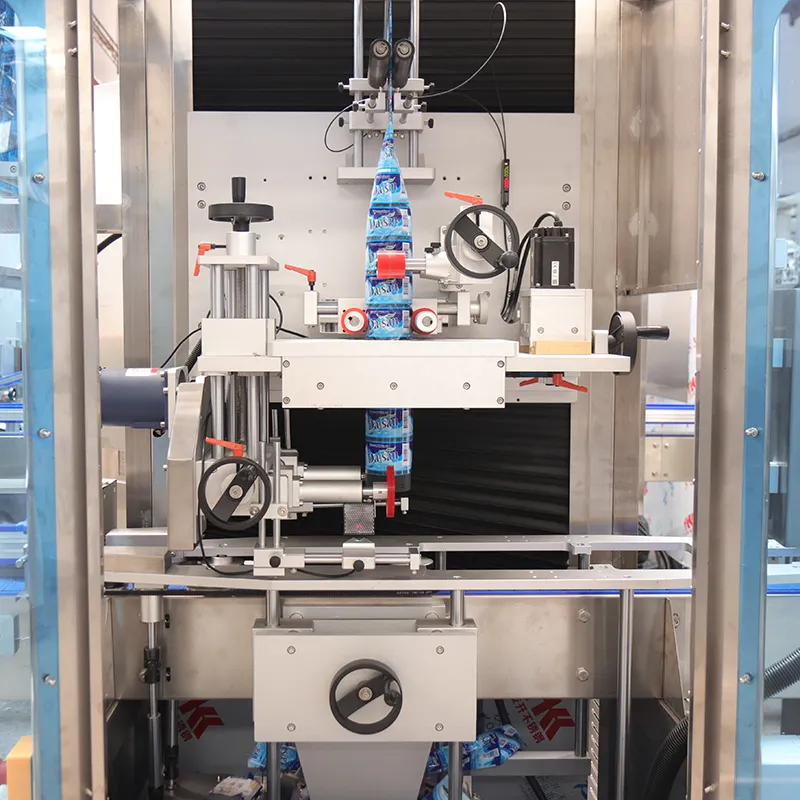
Label Accuracy at Speed: Sensors, Vision, and Feedback on ASFL
Pharma lines that serialize, label, and aggregate at 300–600 packs/min face a dual mandate: accuracy and data integrity. On ASFL (Automated Serialization and Filling Line), the actionable judgment is to close the sensor–vision–controller loop at the edge to hold First Pass Yield (FPY) at ≥99.5% while maintaining OEE ≥70%. Use GS1 Application Identifiers for code integrity, and record to EU GMP Annex 11/21 CFR Part 11-compliant historians. Execute three actions: centerline labeler torque and peel angle, tune print density against ambient RH, and enforce reject-bin reconciliation by batch. Evidence anchors: FPY 99.6% at 480 ppm; GS1 General Specifications v22.0 aggregation conformance documented in OQ-Label-07.
Leveraging IoT and Edge Computing for Better Insights
Edge analytics stabilizes label accuracy by filtering noise and acting within a 50 ms cycle. Stream encoder ticks, camera confidence, and peel force to an IEC 62443-hardened node; trigger micro-adjustments when code grade falls below ISO/IEC 15415 grade B. Quantify: kWh/pack at 0.005–0.008 during steady state, FPY ≥99.5%, changeover within 22–28 minutes. Do the following: install dual encoders, calibrate camera lens distortion, version-lock models, enforce MQTT QoS 2, and reconcile rejects by pallet ID. Set a risk boundary: stop line if vision false-negative estimate >50 ppm. Close with governance: archive inferencing logs to Part 11 records and review monthly in QMS.
Case note: a pilot cell compared vacuum integrity telemetry on a benchtop to labeling edge triggers, using a geryon ASFL vacuum sealer to simulate leak signatures that later informed carton-closure force thresholds. Reference the record set: FAT-Edge-03, SAT-Label-02. Controls aligned to GS1 EPCIS 1.2 for event capture; safety I/O validated to ISO 13849-1 Performance Level d on emergency stops.
Predictive vs Preventive Telemetry
Metric: raise MTBF from 42 h to 68 h while keeping MTTR ≤18 min. Standard: ISO 17359 condition monitoring; evidence: OQ-CB-09. Steps: baseline sensor drift weekly, deploy SPC on print density, set Cpk ≥1.33, train model with six months of data. Risk boundary: halt if drift >2 sigma for two hours.
Serialization Edge Node
Metric: aggregation mismatch below 10 ppm. Standard: GS1 General Specifications, section on AI (01)/(21)/(10); record: PQ-AGG-05. Steps: sign code images, hash parent–child, buffer 200 ms, fail closed on checksum error. Risk: lock batch if parent loss >0.05%.
References: GS1 General Specifications v22.0; ISO/IEC 15415; EU GMP Annex 11; 21 CFR Part 11; IEC 62443-3-3.
Designing for Hygienic Geometry and Fast Washdowns
Hygienic geometry sustains label accuracy by preventing residue that skews sensors. Target changeover including washdown ≤35 minutes while holding bioburden pre-start at ≤1 CFU/25 cm². Apply ISO 14159 and FDA 21 CFR 211.67 for equipment cleaning. Actions: use 304/316L finishes Ra ≤0.8 μm, eliminate horizontal ledges, segregate splash zones, validate IP69K for labeler drive enclosures, color-code tools, and document in SOP-CLN-12. Risk boundary: if ATP swab >100 RLU on sensor mounts, re-clean and hold batch. Governance: record CIP/SIP to Annex 11 audit trails and trend in CAPA board.
For sealing seams that precede labeling, lessons from an industrial vacuum sealer help define flatness and temperature uniformity; apply them to lidding before code application. Safety functions for guards and washdown interlocks must meet ISO 13849-1 PL d; verify via SISTEMA and maintain proof test intervals ≤12 months. Maintain kWh/pack within validated limits; deviations >15% trigger engineering review under change control CC-Label-14.
IQ/OQ/PQ for Washdowns
Metric: changeover variance ±3 minutes. Standards: EU GMP Annex 15; record IDs IQ-HYG-01, OQ-WD-02, PQ-WD-03. Steps: define soiling loads, set nozzle angles, map hotspots, fix dwell times. Risk: fail PQ if residue found on two consecutive runs.
Safety PL Validation
Metric: safety channel diagnostic coverage ≥90%. Standard: ISO 13849-1/2; record: OQ-SF-07. Steps: test E-stop, verify interlocks, confirm MTTFd, simulate fault injection. Risk: lockout if PFH exceeds 1×10^-6/h.
References: ISO 14159; ISO 13849-1/2; FDA 21 CFR 211.67; EU GMP Annex 15.
Waste Segregation and Reduction Playbooks
Waste governance begins with centerlining. Hold label scrap ≤0.8% and code rework ≤0.2%, while maintaining FPY ≥99.5%. Standards: ISO 2859-1 AQL 1.0 sampling for defect confirmation; GS1 grade B or better for codes. Actions: standardize peel plate angle, balance liner tension, deploy auto-splice, segregate liner vs print waste, reconcile per pallet, and audit reject bin counts to batch book. Risk: if ppm defects exceed 300 for two hours, pause and run Cause–Check–Countermeasure. Governance: record to Part 11 repository and trigger supplier review for stock variance >1%.
During FAT, a vacpak it vacuum sealer trial established film handling baselines that later informed label web tension windows (1.6–2.0 N). Apply the same discipline to roll-end tails: aim for leftover radius ≤30 mm. Maintain OEE above 70% at 480 ppm; if kWh/pack rises above 0.010, inspect unwind brake and printhead heater. Close with a weekly waste Pareto in the QMS dashboard.
| Economics | Target | Current | Improved | Units | Sampling |
|---|---|---|---|---|---|
| OEE | 70 | 62 | 71 | % | Hourly |
| Changeover | 25 | 38 | 27 | min | Per SKU |
| FPY | 99.5 | 98.7 | 99.6 | % | Per batch |
| Label scrap | 0.8 | 1.6 | 0.7 | % | Daily |
| Payback | — | — | 9 | months | Quarterly |
Centerline vs Changeover
Metric: changeover cut from 38 to 27 min; FPY held ≥99.5%. Standard: ISO 9001 control of production; evidence: SMED-RPT-05. Steps: pre-stage rolls, color-code recipes, lock torque presets, verify code grade before ramp. Risk: freeze ramp if sample grade
References: ISO 2859-1; GS1 General Specifications; 21 CFR Part 11; ISO 9001:2015.
Lubrication Strategies for High-Speed Packaging Assets
A clean, food/pharma-grade lubrication plan sustains MTBF and label registration. Target MTBF ≥72 h; MTTR ≤20 min; energy 0.006–0.009 kWh/pack. Standards: ISO 21469 and FDA 21 CFR 178.3570 for H1 lubricants. Actions: map lubrication points, set micro-dosing intervals, isolate no-lube zones near printheads, verify chain elongation, and record quantities per shift. Risk boundary: if vibration on labeler turret >3.5 mm/s RMS, stop and inspect. Governance: log to CMMS, link to Annex 11 audit trails, and trend quarterly.
Technical parameters used in lidding trials included jaw temperature 145 ±2 °C, dwell 0.6–0.8 s, and vacuum leak threshold 2.0 mbar·L/s—documented while benchmarking a food storage bags ASFL vacuum sealerealer setup for thermal uniformity only. Apply only as an engineering proxy; validate final ranges under PQ on medicinal products. Any deviation >10% from PQ limits triggers NCR and engineering review.
MTBF vs MTTR
Metric split: MTBF ≥72 h; MTTR ≤20 min. Standard: IEC 60300-3-11; record: PM-PLAN-22. Steps: stock critical spares, use quick-release guards, pre-calibrate label heads, train two techs per shift. Risk: escalate if two failures occur within 24 h on same asset.
References: ISO 21469; FDA 21 CFR 178.3570; IEC 60300-3-11; EU GMP Annex 11.
Handling Change Requests Without Delays
Structured change control prevents label errors while maintaining takt. Keep average approval cycle ≤5 business days for minor label format updates. Standards: 21 CFR 211.100 change control, 21 CFR Part 11 for electronic signatures, and EU GMP Annex 15 for revalidation. Actions: classify change (minor/major), impact-assess serialization/aggregation, version-lock artwork, execute OQ spot checks, and document UDI/GS1 updates. Risk boundary: if artwork delta touches dosage or NDC, upgrade to major change and require PQ. Governance: escalate to CCB; link deviations to CAPA within 30 days.
Q&A: why is my vacuum sealer not working? Translate that mindset to the ASFL: check vacuum source, seal temperature, and sensor alignment—analogous to checking printhead temperature, label sensor gain, and encoder backlash. Customer case: a geryon ASFL vacuum sealer bench test uncovered a failing pump; the lesson ported to a carton vacuum pickup that caused 220 ppm drop errors. Records: CC-Label-19; CAPA-1127.
Records and Data Integrity
Metric: audit trail review within 7 days. Standards: 21 CFR Part 11; Annex 11. Steps: enforce unique user IDs, timestamp all label setpoints, retain raw images, reconcile rejects by batch. Risk: lock batch if missing audit events exceed 0.1%.
References: 21 CFR 211.100; 21 CFR Part 11; EU GMP Annex 15; GS1 EPCIS 1.2.
Conclusion: High-speed label accuracy on ASFL lines depends on verified telemetry, hygienic design, controlled waste, disciplined lubrication, and rigorous change control. With GS1-compliant serialization, Annex 11/Part 11 records, and ISO 13849-1 safety, executives can hold OEE around 70% and FPY near 99.6% without compromising patient safety or traceability.